Scientist-led conferences at Harvard, Stanford and MIT
-
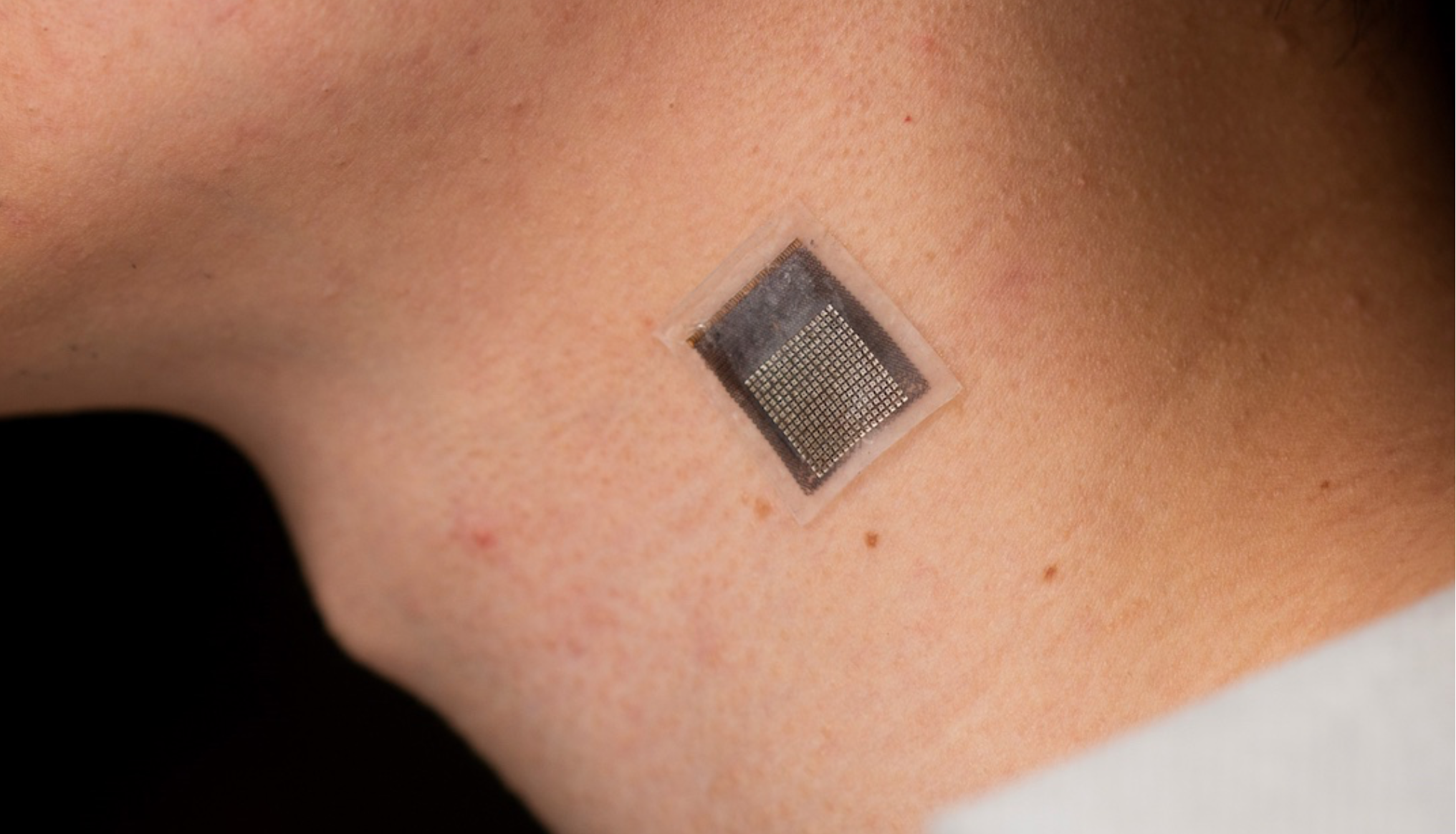
Wearable sensor evaluates human tissue stiffness
Sheng Xu and colleagues have developed a wearable, stretchable device that non-invasively evaluates the stiffness of human tissue, at an improved penetration depth, and for a longer period than, existing methods. An ultrasonic array facilitates serial, non-invasive, three-dimensional imaging of tissues, four centimeters below the surface of human skin, at a spatial resolution of 0.5…
-

Donanemab slowed memory decline by 35%, disease progression by 39%, in Alzheimer’s trial
Patients who received Eli Lily’s monthly donanemab infusion in an 18-month study demonstrated a 35% slower decline in memory, thinking, and ability to perform activities of daily living, and were 39% less likely to progress to the next stage of the disease. Brain plaque was also reduced significantly. The risk of the drug is brain…
-
Pain management via implanted, bioresorbable drug delivery device
John Rogers has developed a wireless, self-powered, bioresorbable implant for programmed drug delivery, enabling precision pain management. When exposed to an external light source, the implant’s wavelength-sensitive phototransistor opens a gate, releasing a pre-loaded reservoir of a drug into the body. It dissolves after the regimen is complete, eliminating the need for surgery to remove…
-

Music improves working memory in study of seniors
A study led by Damian Marie and UNIGE, HES-SO Geneva, and EPFL colleagues showed the effect of music on working memory decline. 132 healthy retirees from 62 to 78 years of age, who had not taken any lessons for at least six months, were assigned to two groups — piano practice, and active listening. According…
-
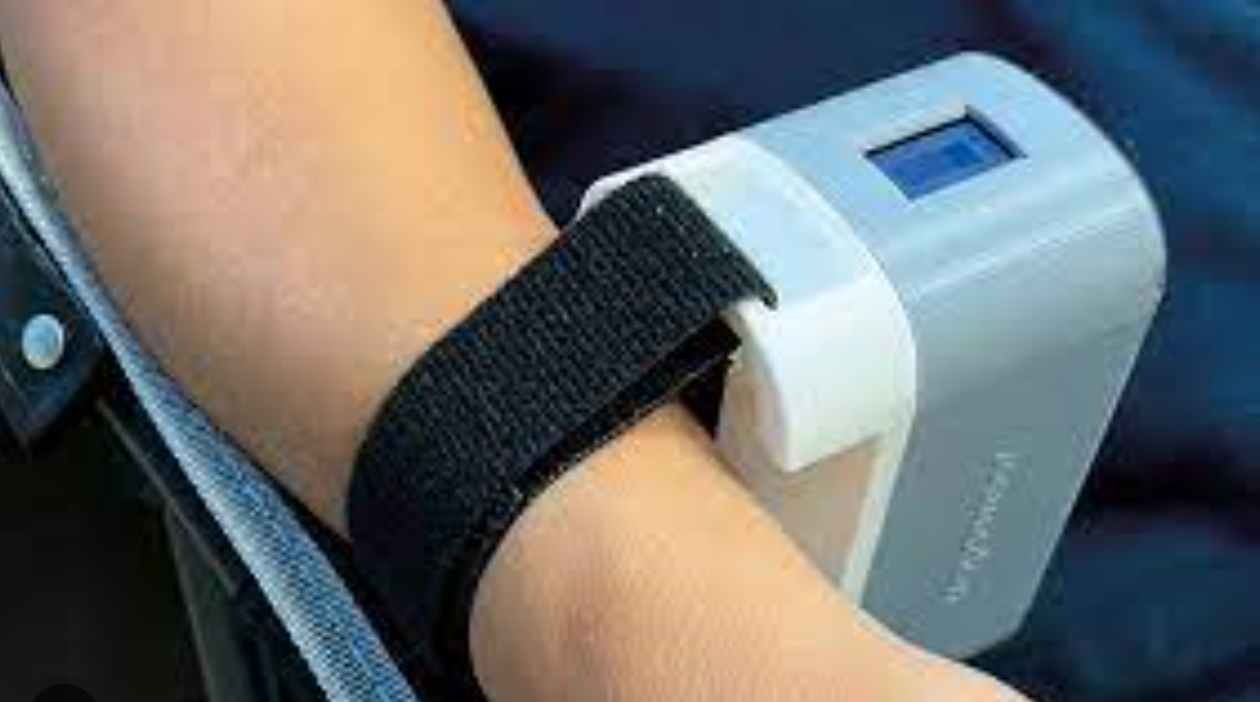
Proof of concept wrist sensor could detect heart-vessel blockage in 3-5 minutes
UW’s Graham Nichol and Harborview Medical Center colleagues studying the reliability of a troponin-detecting, wrist-worn sensor in arriving cardiac arrest patients. Identifying heart-vessel blockage quickly is crucial to ensuring rapid, appropriate intervention. Traditional EKG diagnosis can lack accuracy, and blood testing for troponin can take hours. The novel “Tropsensor,” being commercialized by rce, is designed…
-
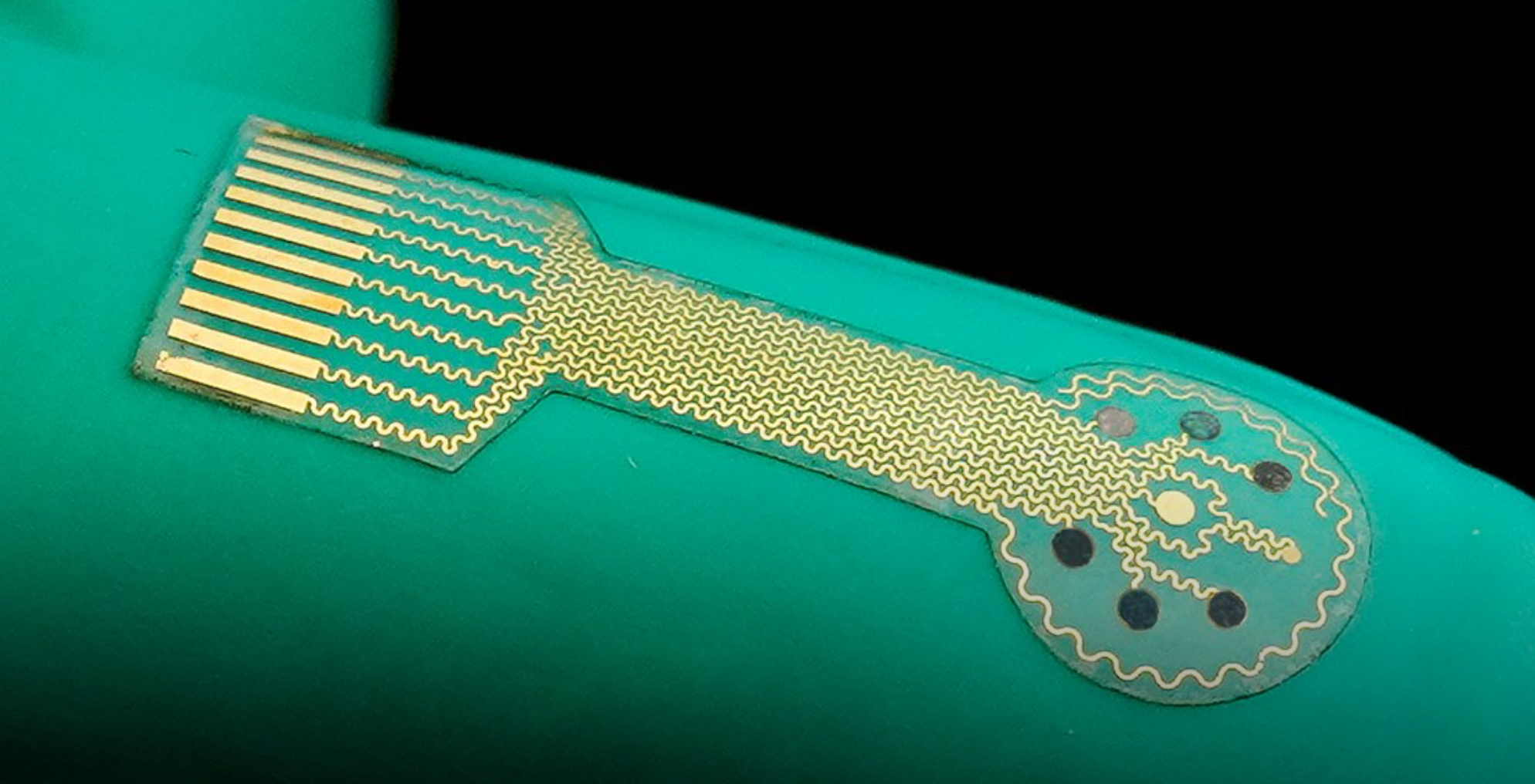
Smart bandage based closed loop wound monitoring and treatment system
Caltech professor Wei Gao has developed a smart bandage for chronic wounds, made from a flexible and stretchy polymer containing embedded electronics and medication. The electronics allow the sensor to monitor uric acid, lactate, pH level and wound temperature, indicating inflammation or bacterial infection. Data from the wound is transmitted wirelessly to the patient or…
-
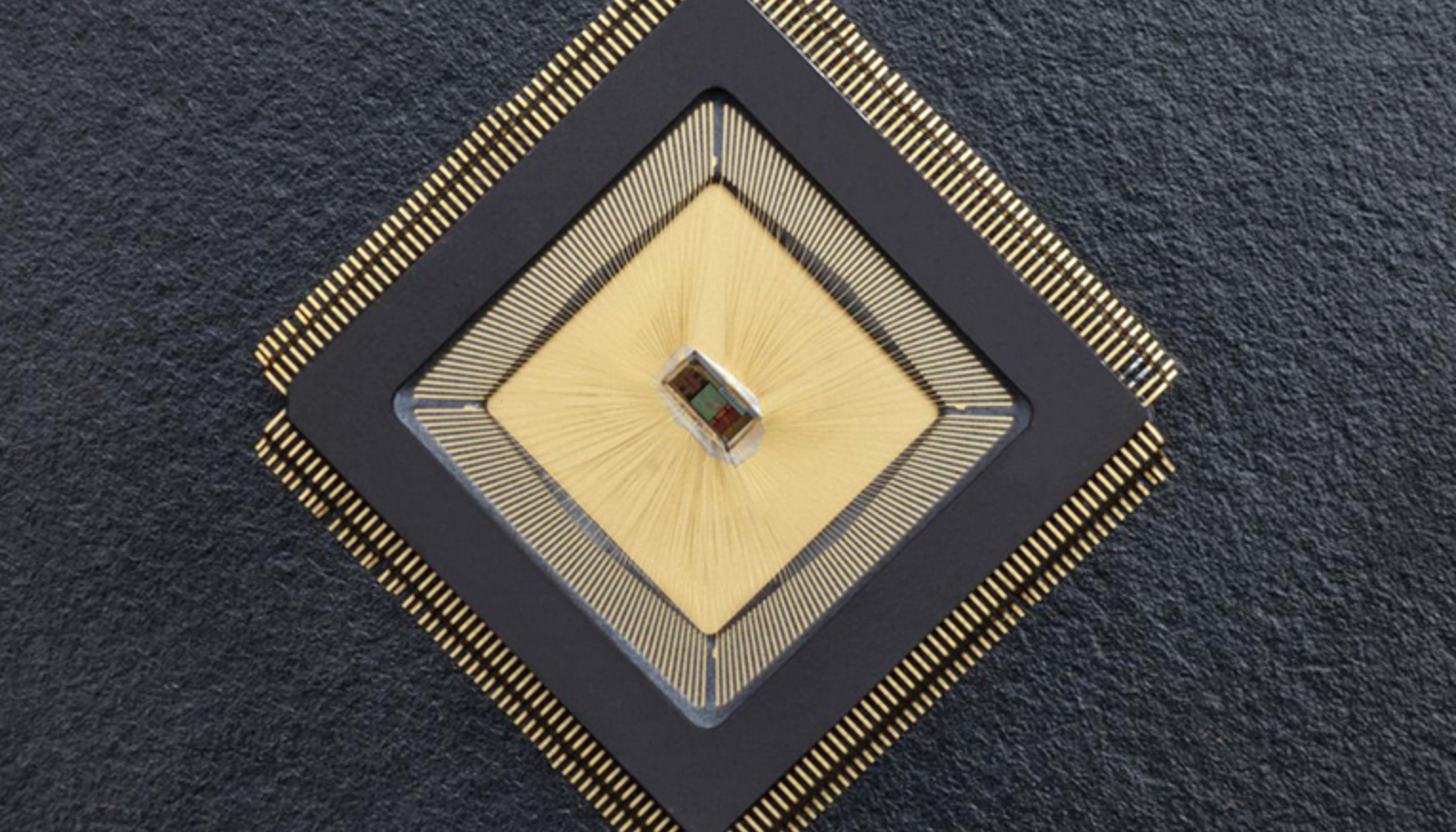
Closed-loop sensor/stimulation system to detect, reduce neurological events
For the first time, there is an autonomous sensing and stimulating unit sitting in a specific brain region and ensuring that neuronal activity in that region is controlled. The closed-loop system is called NeuralTree and was developed by Mahsa Shoaran and EPFL colleagues, to overcome the lack of control caused by epileptic activity or tremor,…
-
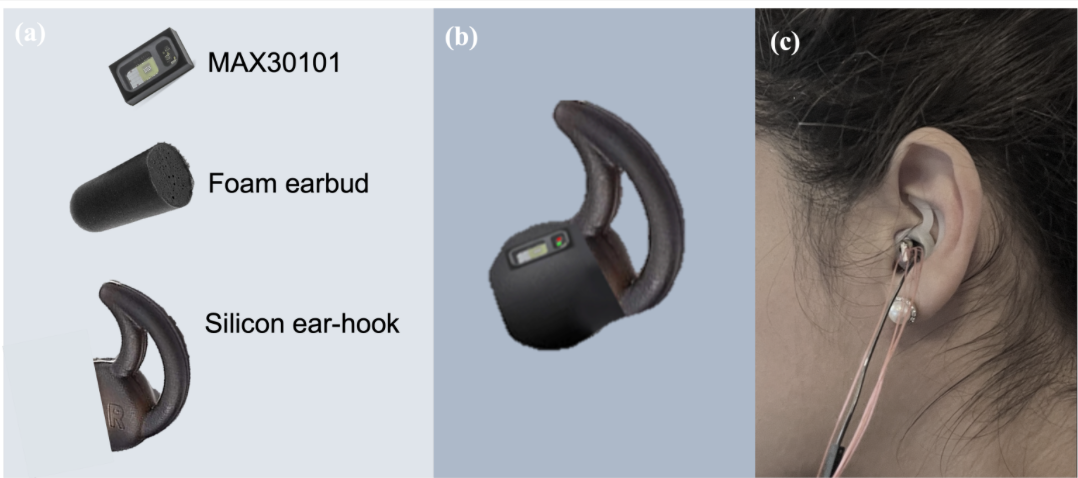
In ear PPG used to measure blood glucose levels in proof of concept study
Danilo Mandic and Imperial College colleagues have developed a novel in-ear PPG device for continuous blood glucose level measurement, using the infrared wavelength of a pulse oximeter. In a recent proof of concept study, non-diabetic, pre-diabetic, type I diabetic, and type II diabetic states were considered. Recordings spanned 9 days, in both fasting and post carbohydrate consumption…
-

AI reconstructs viewed images
Yu Takagi, Shinji Nishimoto and Osaka University colleagues have published a study which demonstrates that AI can read brain scans and re-create largely realistic versions of images a person has seen. Future applications could include enabling communication of people with paralysis, recording dreams, and understanding animal perception, among others. Additional training was used on the existing text-to-image generative…
-
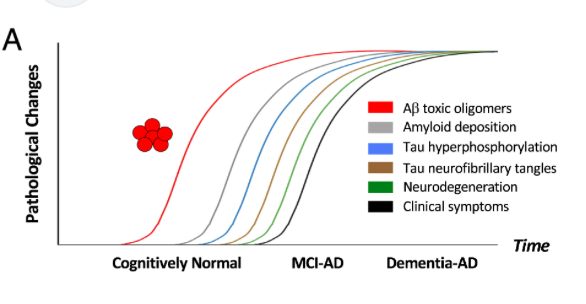
Amyloid beta oligomer blood test could predict Alzheimer’s disease several years in advance
University of Washington’s Valerie Daggett, Dylan Shea, and colleagues, have developed a lab test that can measure levels of amyloid beta oligomers in blood samples. Known as SOBA, the test detected, in a study of 310 subjects, oligomers in the blood of Alzheimer’s patients, but not in most of the control group, which had no…
-
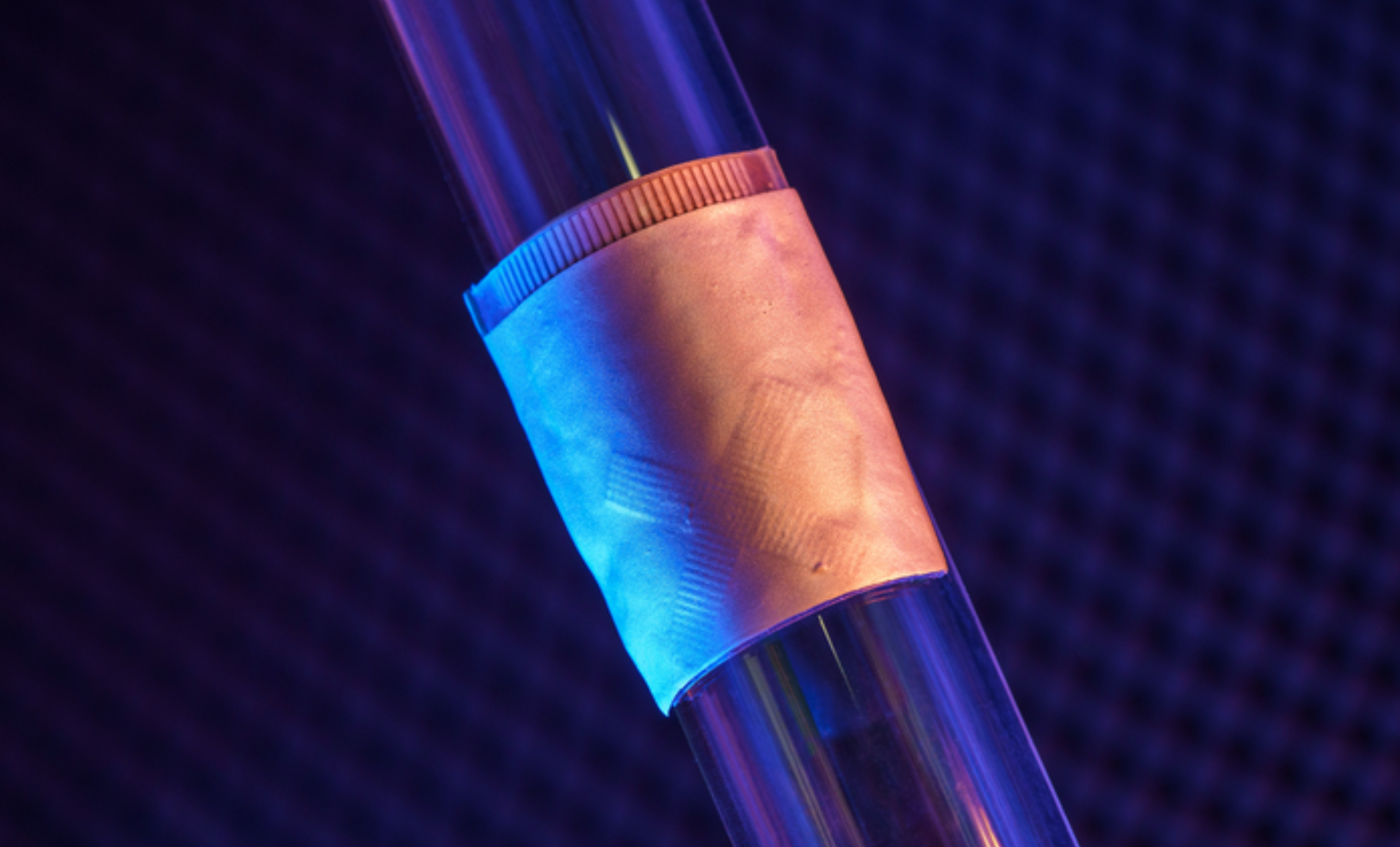
Wearable cardiac ultrasound continuously monitors heart structure, function for 24 hours
UCSD professor Sheng Xu has developed a wearable ultrasound device that can continuously monitor and assess the structure and function of the human heart for 24 hours, during normal daily activity. This could eliminate the need for highly trained technicians and bulky devices. Signs of cardiac diseases are transient and unpredictable, and imaging can detect…
-

Wearable sensor glows when bacteria, toxins detected
David Baker, Fiorenzo Omenetto, and University of Washington and Tufts colleagues have developed a garment-printed biopolymer sensor which detects bacteria, toxins, and dangerous chemicals. To work, a chemical activator must be sprayed after potential exposure. If the target is present, the sensor generates light. The intensity of emitted light provides a quantitative measure of the…
Got any book recommendations?